
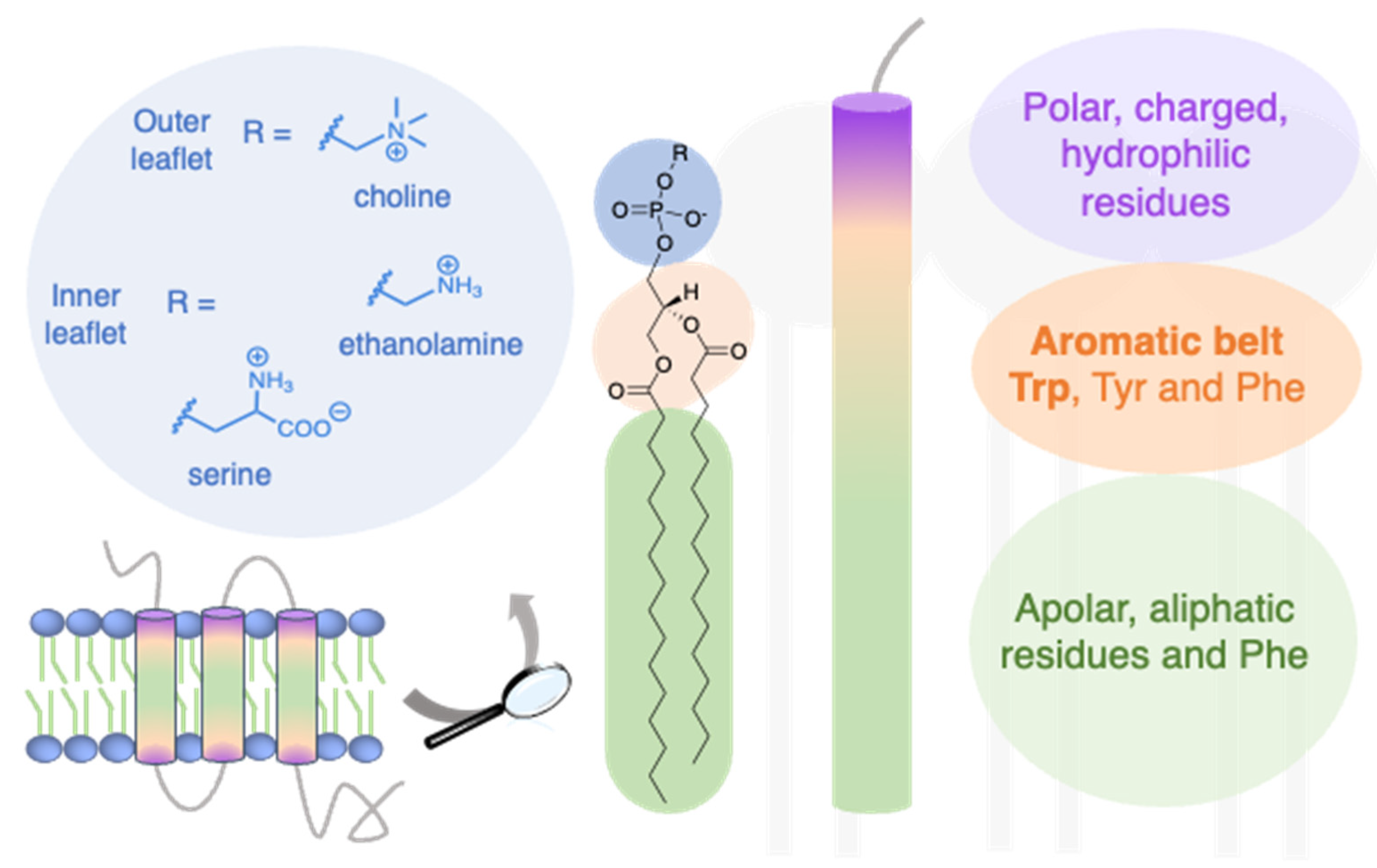
The side chains ( R groups) of such amino acids as phenylalanine and leucine are nonpolar and hence interact poorly with polar molecules like water. Increasing salt concentration reduces the strength of ionic binding by providing competing ions for the charged residues. Link to example showing how all serum proteins become negatively-charged at a pH of 8.6. The result: Again the net charge on the molecule changes (it becomes more negative) and, again, many of the opportunities its R groups have for electrostatic interactions with other molecules or ions are altered.
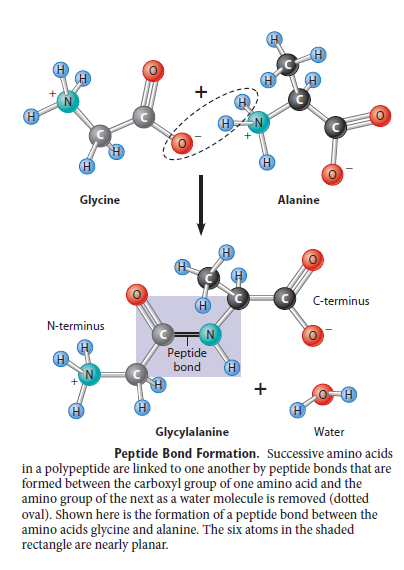
H + bind to the unoccupied pair of electrons on the N atom of the amino (NH 2) groups of lysine (Lys) and arginine (Arg) giving them a positive charge. H + bind to the carboxyl groups (COO -) of aspartic acid (Asp) and glutamic acid (Glu), neutralizing their negative charge, and Ionic interactions are highly sensitive to
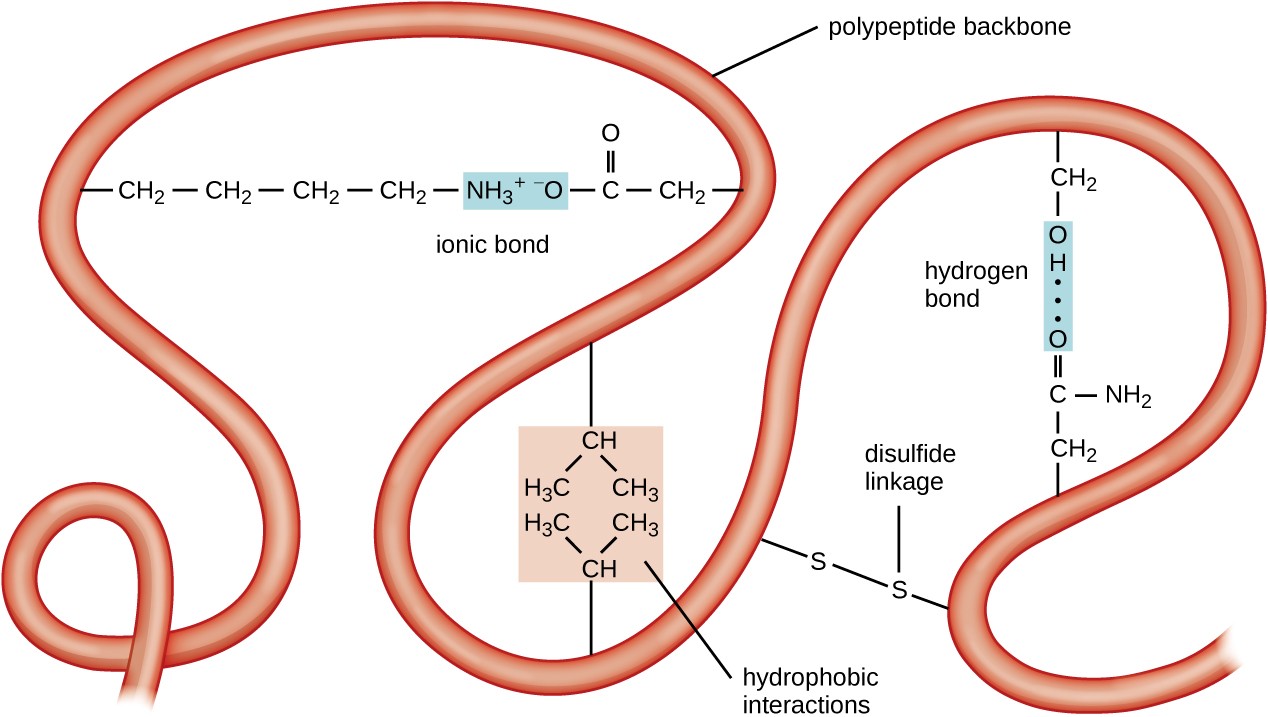
There are three principle kinds of noncovalent forces.Īt any given pH, proteins have charged groups that may participate in binding them to each other or to other types of molecules.įor example, as the figure shows, negatively-charged carboxyl groups on aspartic acid (Asp) and glutamic acid (Glu) residues may be attracted by the positively-charged free amino groups on lysine (Lys) and arginine (Arg) residues. permits the assembly of such macromolecular machinery as.some hormones) to bind to their receptor enables transcription factors to bind to DNA.enables transcription factors to bind to each other.enables antibodies to bind to their antigen.enables enzymes to bind to their substrate.folds polypeptides into such secondary structures as the alpha helix and the beta conformation.holds the two strands of the DNA double helix together ( hydrogen bonds).Individually weak but collectively strong.


 0 kommentar(er)
0 kommentar(er)
